Lithium metal oxide structures include cobalt, manganese, and nickel metals. The presence and proportion of these metals vary according to the type of battery and where it is used. Lithium metal oxides come in forms such as LiCoO2, LiMn2O4 and Li (NixMnyCoz) O2.
Lithium ion is named because of its active ingredients.
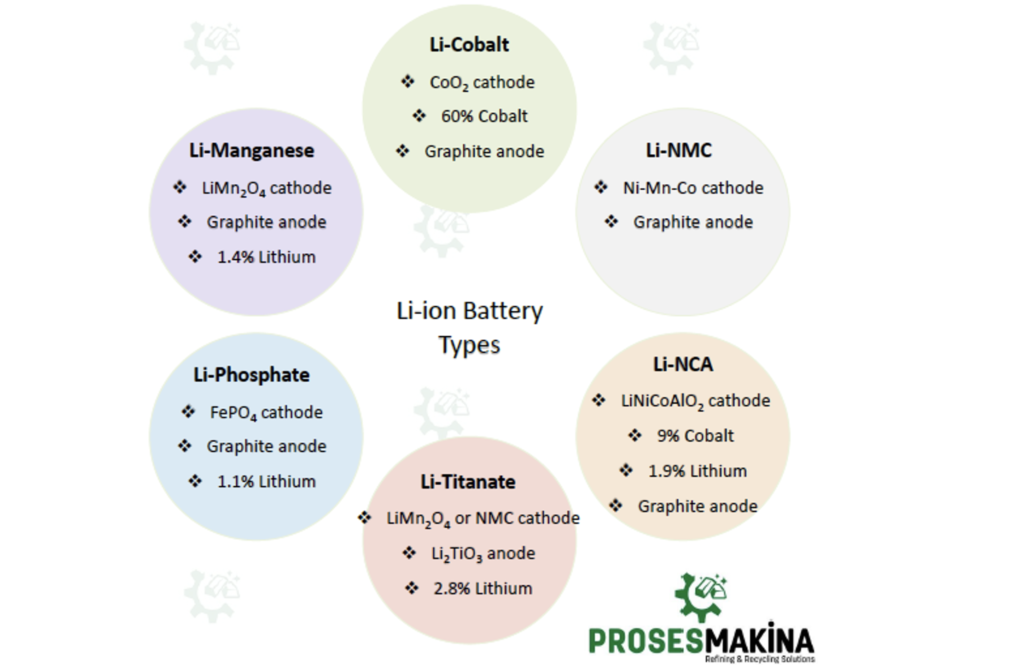
- Lithium Cobalt Oxide, since 1991, one of the most common Li-ions, has the chemical formula LiCoO2 and abbreviation LCO. Li-cobalt can also be used for this battery. Cobalt is the main ingredient that gives this battery its character.
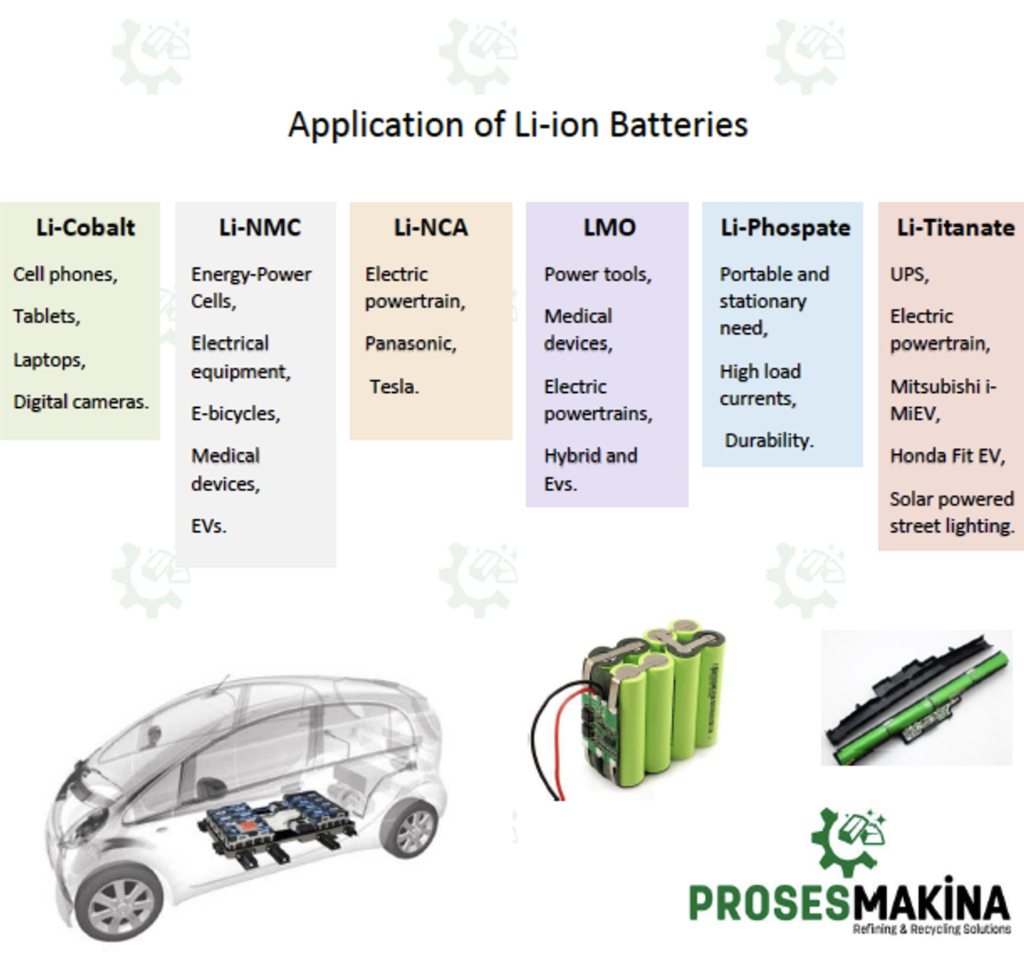
Li-cobalt battery is used in cell phones, tablets, laptop computers and digital cameras. The battery consists of a cobalt oxide cathode (about 60% Co) and a graphite carbon anode. The disadvantage of Li-cobalt is a relatively short life, low thermal stability, and limited load capacity.
Newer systems that contain nickel, manganese and / or aluminum to improve longevity, loading capacities and cost.
- Lithium Manganese Oxide, since 1996, has the chemical formula LiMn2O4 and abbreviation LMO. Li-ion cell is made with lithium manganese oxide as cathode material and graphite anode.
Li-manganese is used for power tools, medical devices, electric powertrains, hybrid, and electric vehicles. Li-manganese has high power but less capacity than li-cobalt but safer. It is often mixed with NMC (lithium nickel manganese cobalt oxide) to improve its performance. This combination brings out the best in each system and the LMO (NMC) is chosen for most electric vehicles such as the Nissan Leaf, Chevy Volt, and BMW i3. The LMO part of the battery, which can be about 30%, provides high current boost on acceleration; the NMC part provides the long driving range.
While pure Li-manganese batteries are no longer common today, they can only be used for special applications.
- Lithium Nickel Manganese Cobalt Oxide, since 2008, has the chemical formula LiNiMnCoO2 and abbreviation NMC. One of the most successful Li-ion systems is the nickel-manganese-cobalt (NMC) cathode combination and graphite anode.
Similar to li-manganese, these systems can be adapted as Energy Cells or Power Cells. NMC’s success is due to the combination of nickel and manganese. Nickel is known for its high specific energy but poor stability; manganese has the advantage of forming a spinel structure to achieve low internal resistance but offers a low specific energy. Combining metals increases each other’s strength.
NMC is the battery of choice for electrical equipment, electric bicycles, medical devices, EVs and other electric powertrains.
- Lithium Iron Phosphate, since 1996, has the chemical formula LiFePO4 and abbreviation LFP, Li-phosphate or LIP. Li cell is made with phosphate as cathode material and graphite anode.
Its field of application is portable and stationary need, high load currents and durability. Very flat voltage discharge curve, but low capacity. One of the safest Li-ions. It is used for special markets. Elevated self-discharge. It is primarily used for energy storage.
- Lithium Nickel Cobalt Aluminum Oxide, since 1999, has the chemical formula LiNiCoAlO2 and abbreviation NCA or Li-aluminum.
NCA is used in medical devices, industrial, electric powertrain mainly used in Panasonic and Tesla. The battery consists of LiNiCoAlO2 cathode (9% Co) and graphite anode.
Lithium nickel cobalt aluminum oxide battery, or NCA, offering high specific energy, very good specific power, and long life. NCA is a further development of lithium nickel oxide; adding aluminum provides more stability to the chemistry.
- Lithium Titanate, since 2008, has the chemical formula Li2TiO3 and abbreviation LTO or Li-titanate. Cathode can be lithium manganese oxide or NMC; Li2TiO3 (titanate) is anode.
Li-titanate replaces the graphite in the anode of a typical lithium-ion battery. The cathode can be lithium manganese oxide or NMC. The cycle count is said to be higher than that of a normal Li-ion. Li-titanate is safe, has excellent low temperature discharge properties.
UPS, electric powertrain (Mitsubishi i-MiEV, Honda Fit EV), solar powered street lighting usage areas.
Lithium-ion batteries contain precious metals and other materials that can be recycled, processed, reused, and critical to reserve. But the recycling rate is very low. Proses Makina Company’s R&D studies continue to improve the lithium-ion battery recycling system and make it more efficient.
Proses Makina Company has precious metal and rare metal recovery and refining technology from electronic waste, catalytic converter, and also primary production lines from scrap jewelery or ore. R&D studies continue to improve the lithium-ion battery recycling system and make it more efficient. It manufactures and installs turnkey facilities with industrial scale, efficient, high technology, and necessary process know-how.